SOMATIC EVOLUTION ACROSS SPECIES
Why study somatic evolution across species?
Ageing
There is enormous diversity in the rates of ageing and lifespans across species. Comparing somatic mutational processes and clonal dynamics in species with diverse lifespans could provide insights into the complex factors that contribute to ageing and potentially guide future interventions for age-related diseases.
Cancer
Cancer risk does not increase proportionally with lifespan or body-size across species. This observation, known as ‘Peto’s Paradox’, suggests that larger, longer-lived species have evolved superior cancer suppression mechanisms. Studying somatic mutation and clonal dynamics in these species could lead to the discovery of their cancer resistance mechanisms, opening the door for novel therapeutic strategies.
Environmental Monitoring
Environmental pollutants are a significant contributor to early mortality, especially in underprivileged communities. The distinctive mutational signatures created by many pollutants could be leveraged for genomic surveillance of sentinel species, offering a fast and effective tool to monitor the presence and impact of pollutants, similar to the ‘canary in the coal mine’.
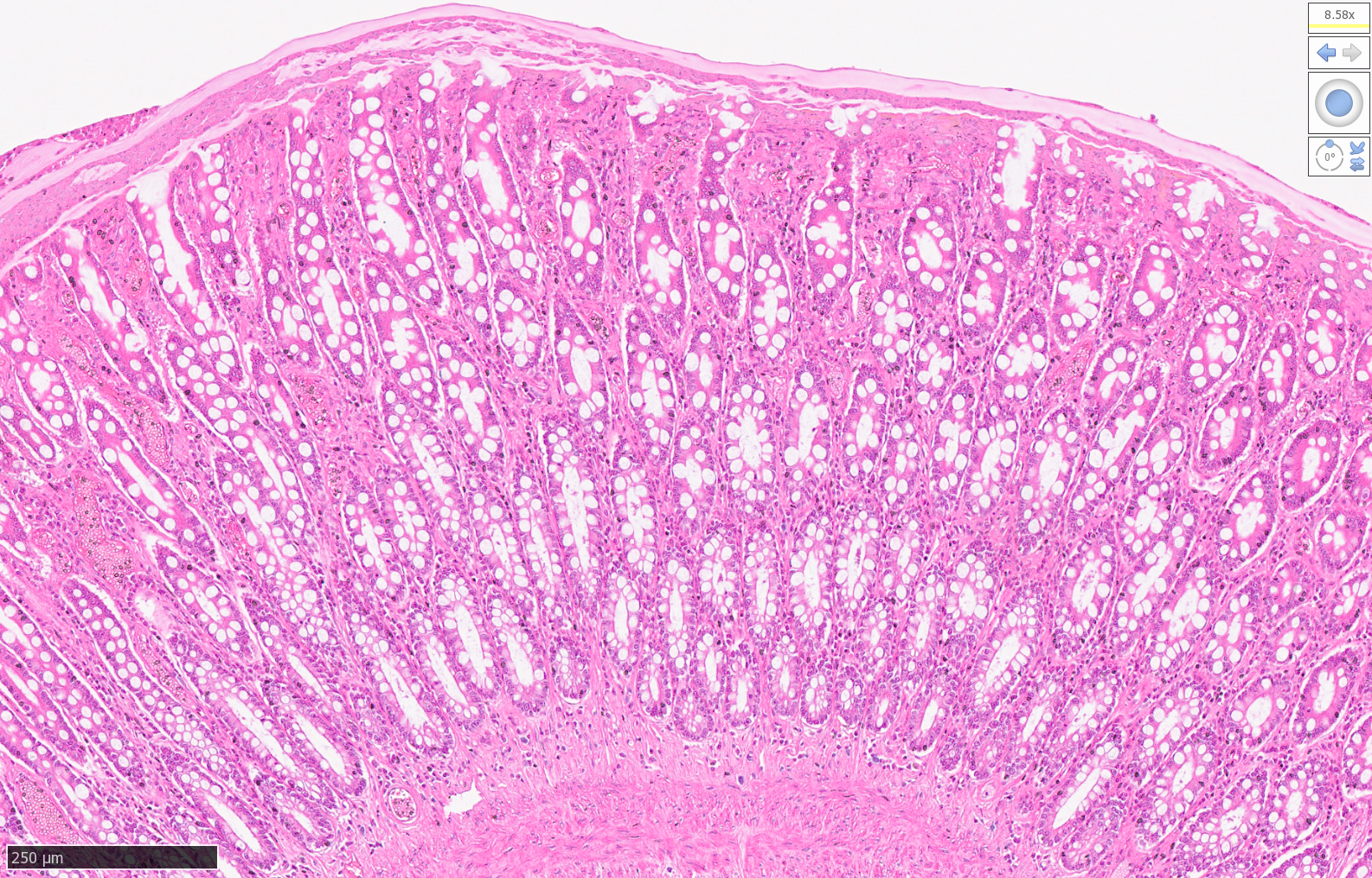
High Resolution Scans of Histological Sections Prepared for Laser Capture Microdissection:

Naked Mole-Rat Intestine

Lion Intestine
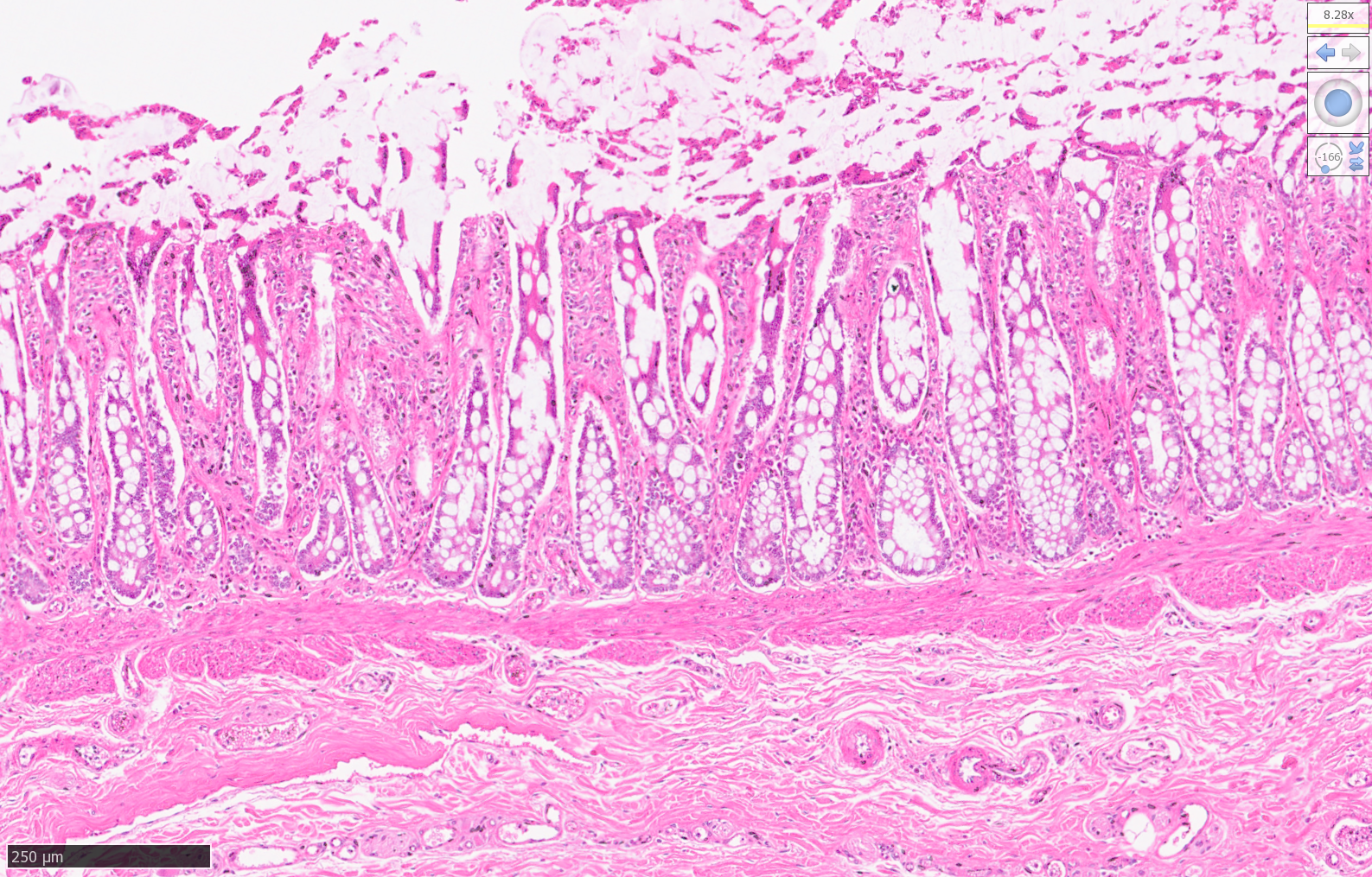
Tiger Intestine

Harbour Porpoise Intestine

Mouse Intestine
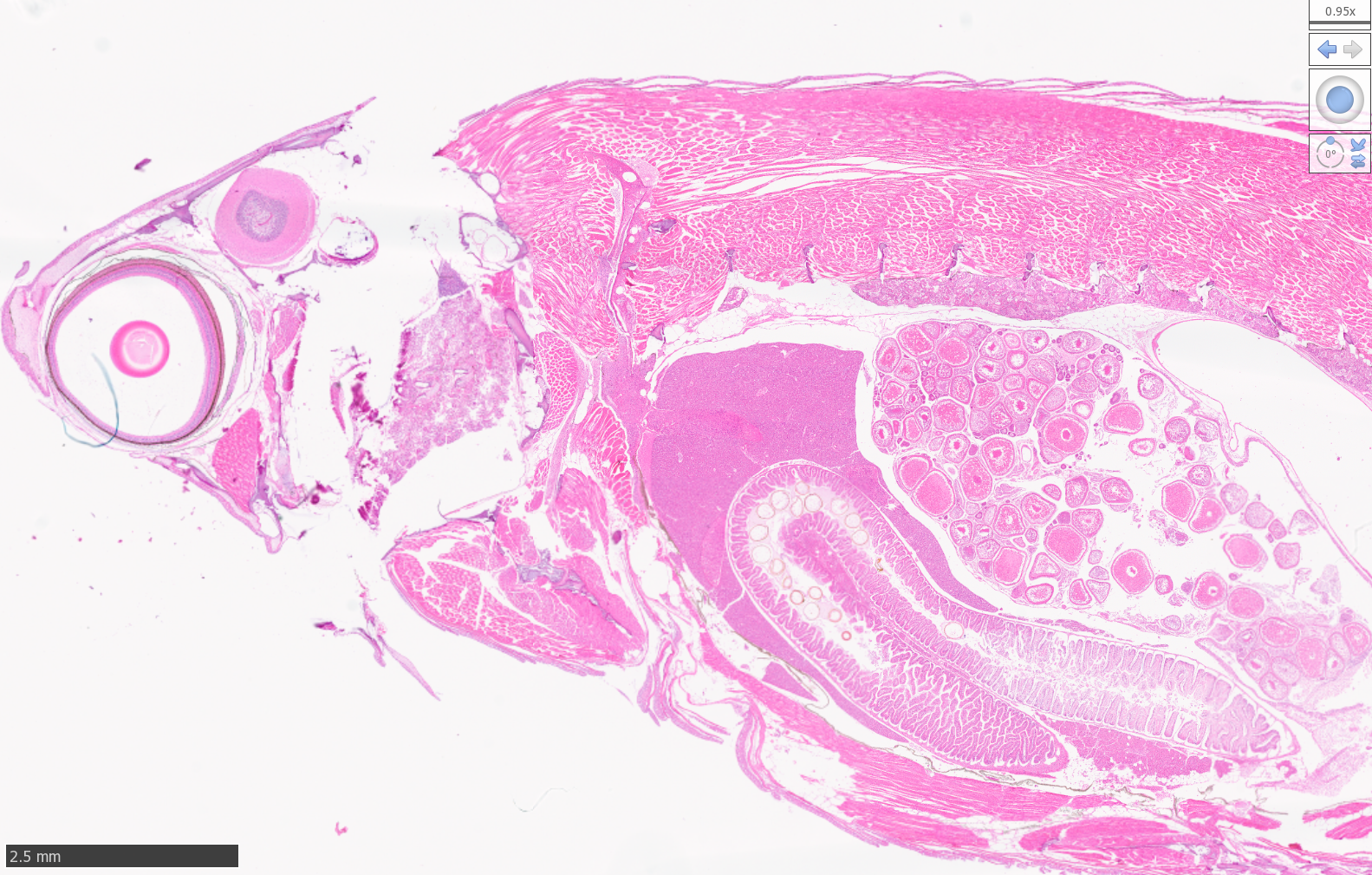
Zebrafish
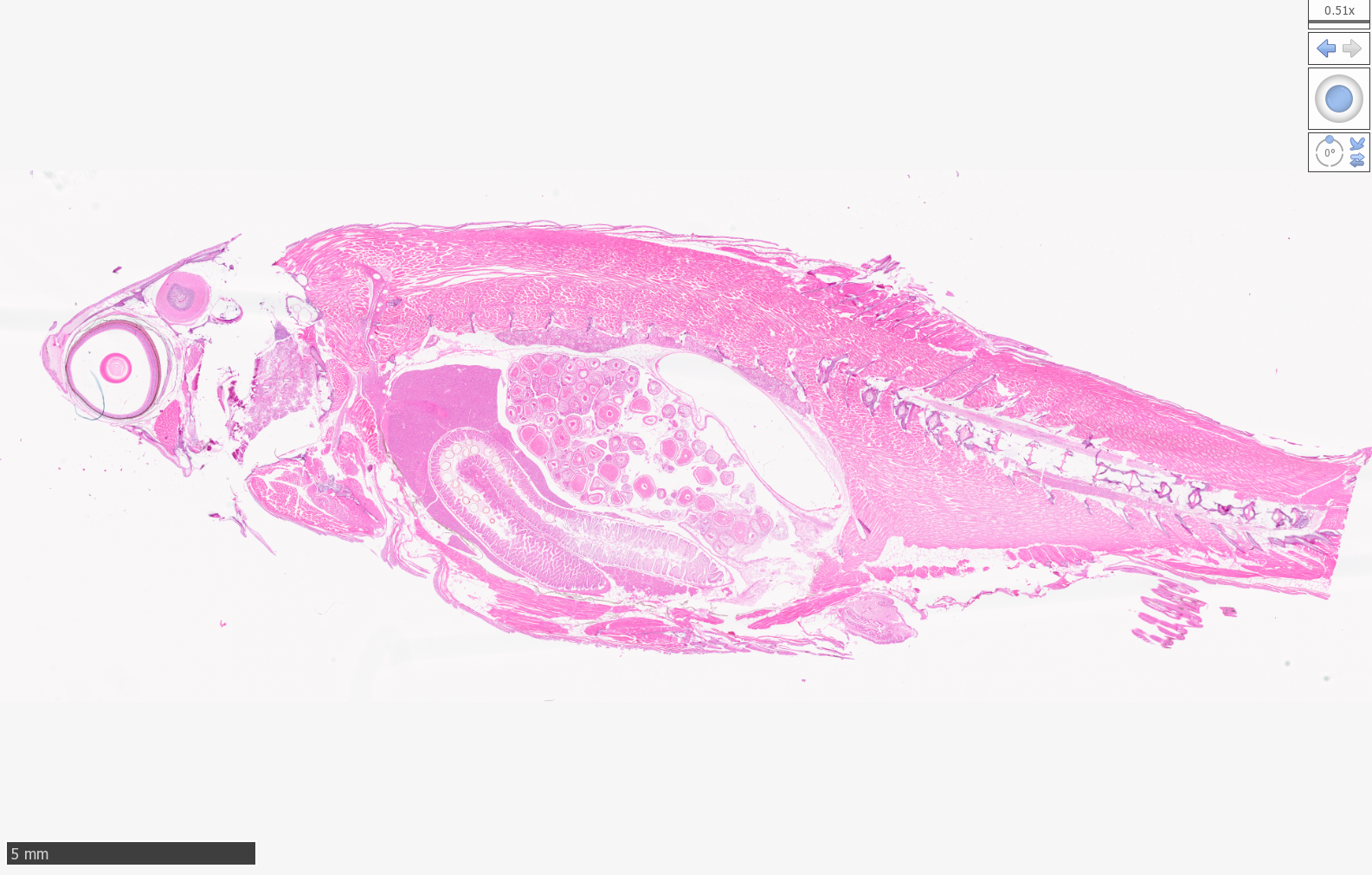
Zebrafish
Selected Publications
Cagan et al, 2023 ‘Somatic mutation rates scale with lifespan across mammals’ Nature (2022)
We developed a pipeline integrating histology, laser capture microscopy and low-input genome sequencing to compare somatic mutational processes in 208 intestinal crypts from 56 individuals in 16 mammalian species with diverse lifespans and body-sizes. Somatic mutations were dominated by endogenous processes common across species. Strikingly, a strong inverse relationship between somatic mutation rates and lifespan suggests that somatic mutation rates are evolutionarily constrained, partially explain variation in cancer risk across species, and may play a role in ageing.
This study compared the mutational landscape in 29 cell types across the human body, including direct quantification of paternal de novo mutation rates from spermatogonial stem cells. Two ubiquitous mutational signatures accounted for the majority of acquired mutations in most cell types, though their absolute and relative contributions varied substantially. Fascinatingly, spermatogonia, the cells responsible for most genetic variation in human populations, exhibited the lowest mutation rates, implying that germ cells possess superior mechanisms of genomic maintenance compared to their somatic counterparts.

Somatic Evolution

Peto's Paradox & the Impossibility of Whales

Alternative Journal Cover
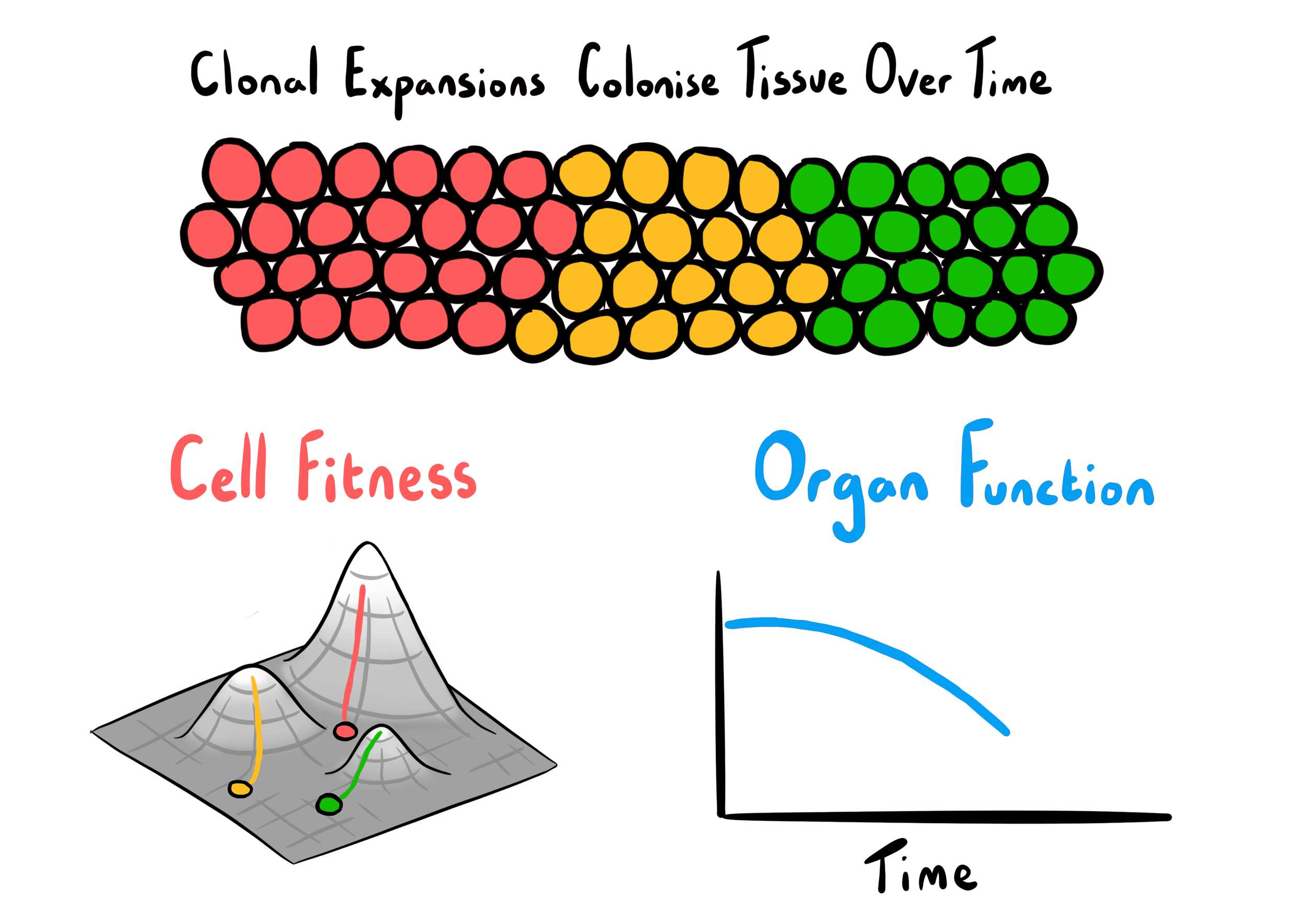
Clonal dynamics and ageing - a hypothesis
Employment
2023-Present
Assistant Professor
Departments of Genetics, Pathology and Veterinary Medicine
University of Cambridge
2022-2023
Staff Scientist
Wellcome Sanger Institute, UK
Supervisor: Inigo Martincorena
2017-2022
Postdoctoral Researcher
Wellcome Sanger Institute, UK
Supervisor: Inigo Martincorena
2010-2017
PhD Evolutionary Genomics
Max Planck Institute for Evolutionary Anthropology, Germany
Supervisor: Svante Pääbo